More than 300 staff of a HCMC-based company have worked overtime for over six months on the first Covid-19 vaccine approved for human trials in Vietnam.
A representative of the Nanogen Pharmaceutical Biotechnology Company said they began researching vaccine production in March as the Covid-19 epidemic turned into a pandemic.
In Vietnam, the situation had worsened since early March as many Vietnamese nationals returning from the U.S. and Europe and foreigners coming from the same regions were infected with novel coronavirus, prompting the government to impose a national social distancing campaign for more than three weeks and suspend all international flights.
In June, the Ministry of Science and Technology officially asked Nanogen to implement two projects: production of a vaccine and Covid-19 treatment antibodies.
For more than six months, over 300 specialists of the company have worked non-stop on the vaccine project. The basic stages have been completed, including protein synthesis, purification, finalization of the finished formula, repeated tests of toxicity and other tests on mice and monkeys.
Initially, the company had two candidates: a sub-unit vaccine based on SARS-CoV-2’s spike protein, and the virus like particles (VLP) vaccine, using recombinant technology. To expedite availability of the vaccine, Nanogen chose to produce the sub-unit version, whose finished product is Nanocovax, the human trial of which are expected to begin December 17.
The health ministry said the company will cooperate with the Vietnam Military Medical Academy to start recruiting volunteers to participate in the first phase of the human trial from Thursday.
The representative said that the company had independently researched and produced the Nanocovax vaccine. However it had designed its gene segment based on some publications and studies on the gene and protein segments published by prestigious journals, facilitating production of a Covid-19 resistant vaccine.
There are many different strains of the novel coronavirus to be found around the world. The variant in Vietnam spreads rapidly but is less virulent. “We have adjusted the protein fragment suitable to this strain to produce the best immune response,” the representative said.
Nanogen has said that it has tested the vaccine many times on rats and monkeys with good results; and can guarantee its immunogenicity and safety levels.
The vaccine has side effects but these are not significant, the company maintains. Some rats developed itches and suffered from mild irritation at the injection site for about half an hour. The rodents still gained weight, ate and lived normally and showed no toxicity symptoms for a long time after.
The heart, kidneys and lungs of the rats injected with Nanocovax vaccine were all normal without any damage, test surgeries found.
On Monday, the National Institute of Hygiene and Epidemiology announced the Nanocovax test results on hamsters. The hamsters were vaccinated and then exposed to the novel coronavirus for 14 days. They were not infected and results from lung fluid tests also showed they were negative for the virus. Meanwhile, mice that were not vaccinated tested positive for the novel coronavirus and showed signs of fatigue.
Human trial
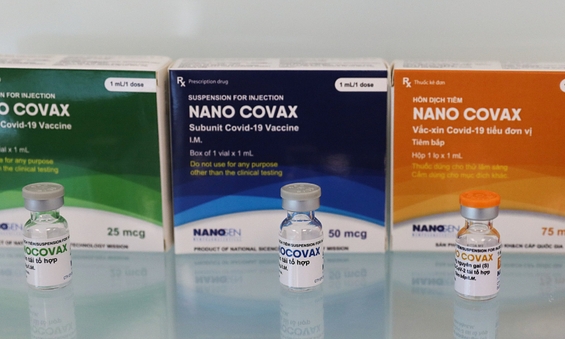
During the first stage of human trial, 60 volunteers will be split into three smaller groups, each receiving three different vaccine dosages: 25 milligrams, 50 milligrams and 75 milligrams.
In a safety measure, the first three people will be vaccinated with 25 mcg of the vaccine and their health assessed 72 hours later. It is after this that the dosage and the number of participants for the next testing session will be decided, the health ministry said Wednesday.
All volunteers during the first phase will receive two intramuscular injections of the vaccine, with an interval of 28 days between the two injections.
Sixty volunteers aged 18-60 without any underlying condition, infectious diseases or allergies are expected to be vaccinated during the first stage.
Nanogen’s Covid-19 vaccine Nanocovax is expected to be priced at a maximum of VND500,000 ($21.62) per dose.
The company representative said that Nanocovax would be reasonably priced and affordable to all Vietnamese, and the company is trying to include the vaccine into the list of drugs covered by health insurance.
Nanocovax is scheduled to go into mass production in May 2021.
Vietnam’s current Covid-19 tally stands at 1,381, including four imported cases confirmed on Wednesday night, raising total active cases to 118. The country has gone nine days without recording a new community transmission.
